Regulated Medical Waste Containers Must

Are reusable containers used to hold regulated medical waste disposed of as regulated medical waste if the containers can t be properly decontaminated.
Regulated medical waste containers must. Rmw is solid waste that comes from medical treatment for animals or humans. Regulated medical wastes are treated or decontaminated to reduce the microbial load in or on the waste and to render the by products safe for further handling and disposal. Treatment of regulated medical waste. Any recognizable human body part organs and tissue or any animal body part organ or tissue contaminated or suspected to be contaminated by a zoonotic disease.
Corrugated boxes should be sealed on the bottom with two inch wide clear packing tape. Does each package of untreated regulated medical waste to be transported off site display the biohazard symbol or have a water resistant label or print on the outside of the container with the. All regulated institutions must complete the medical waste tracking form pdf 19 kb. A warning label that includes the universal biohazard symbol followed by the term biohazard must be included on bags containers of regulated waste on bags containers of contaminated laundry on refrigerators and freezers that are used to store blood or opim and on bags containers used to store dispose of transport or ship blood or.
A warning label that includes the universal biohazard symbol followed by the term biohazard must be included on bags containers of regulated waste on bags containers of contaminated laundry on refrigerators and freezers that are used to store blood or opim and on bags containers used to store dispose of transport or ship blood or. Rmw can include cultures or stocks blood and human waste. A non bulk packaging used as a sharps container must be puncture. Businesses must follow their rules.
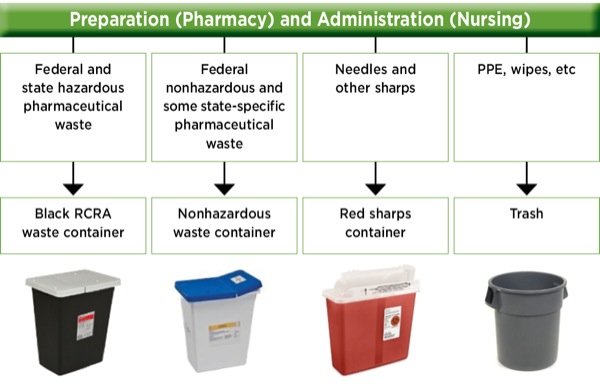
Except as provided in 173 134 c of this subpart non bulk packagings for regulated medical waste or clinical waste or bio medical waste must be un standard packagings conforming to the requirements of part 178 of this subchapter at the packing group ii performance level. Printed text and arrows should differentiate the receptacle s top and bottom. Nys rmw regulatory authority and jurisdiction new york state has provided regulatory oversight of rmw since the early 1980s and has adopted a comprehensive regulatory framework covering all aspects of handling storage treatment and disposal of this waste. Organizations can either use corrugated boxes or specially designed reusable containers to hold rmw.
Non bulk packagings large packagings and non specification bulk outer packagings used for the transportation of regulated medical waste or clinical waste or bio medical waste must be rigid containers meeting the provisions of subpart b of this part. Businesses that generate rmw must package and label waste properly. The us department of transportation usdot governs the disposal of regulated medical waste rmw. Except as provided in 173 134 c of this subpart non bulk packagings for regulated.